Rewiring the grain
Metabolic engineering
Golden Rice technology is based on the simple principle that rice plants possess the whole machinery to synthesise β-carotene, and while this machinery is fully active in leaves, parts of it are turned off in the grain. By adding only two genes, a plant phytoene synthase (psy) and a bacterial phytoene desaturase (crt I), the pathway is turned back on and β-carotene consequently accumulates in the grain.

Fig 1: Pathway overview: carotenoid biosynthesis begins with a small (C5) compound, isopentenyl-diphosphate (IPP) and its isomer dimethylally-diphosphate (DMAPP). Chain elongation by C5 units leads to the formation of the C20-compound geranylgeranyl-diphosphate (GGPP). GGPP is a precursor that can enter several biosynthetic routes, as indicated. The head-to-head condensation of two GGPP molecules produces the first,colourless carotene, phytoene. A series of desaturation reactions lead to the coloured chromophore of lycopene, and subsequent cyclization reactions produce the β- and ε-ionone rings. Oxygenation (hydroxylation, epoxidation) reactions lead then to xanthophylls. Several known pathways branch off at this point, forming biologically important molecules, such as abscisic acid and strigolactones
Carotenoids and their derivatives include a vast number of molecules and accordingly a great number of enzymes and cofactors. Only a small number of carotenoids namely those with at least one unsubstituted β-ionone ring, such as β-carotene have provitamin A activity. Compounds derived from this important pathway include plant hormones, like abscisic acid, the strigolactones and gibberellins. Tocopherols (vitamin E), chlorophylls and quinones employ the pathway intermediate GGPP as a building block for their synthesis.
The underlying science in more detail
All plant tissues that accumulate high levels of carotenoids have mechanisms for carotenoid sequestration, including crystallisation, oil deposition, membrane proliferation or protein-lipid sequestration. It has been shown that lipid accumulation can be a driver of carotenoid formation by acting as a lipophilic sink (Rabbani et al., 1998). The non-carotenogenic starchy rice endosperm, on the other hand, is very low in lipid and apparently lacks any such means for carotenoid deposition. It was also doubtful whether Golden Rice would have the necessary precursors for carotene biosynthesis present and available in the grains, with many believing that the whole, multi-step carotenoid biosynthetic pathway was completely absent from the endosperm.
This explains why a long research phase preceded the achievement of the proof-of-concept for Golden Rice. By the early 1990s, the data accumulated became encouraging enough for Profs Peter Beyer and Ingo Potrykus to gather forces and dare to tackle this feat. Their breakthrough showed that only two transgenes were required to turn Golden Rice into a reality (Ye et al., 2000). The first transgene encodes a plant phytoene synthase (PSY), which utilises the endogenously synthesised geranylgeranyl-diphosphate (GGPP) to form phytoene, a colourless carotene with a triene chromophore (Burkhardt et al., 1997). The second gene encodes a bacterial carotene desaturase (CRTI) that introduces conjugation by adding four double bonds. Between 1993 and 1999, collaborative research between Peter Beyer and Prof Peter Bramley (Royal Holloway College, UK), was funded through EU networks B102-CT-930400, B104-CT97-2077 and FAIR CT96 1633. Working with genetically modified tomatoes, Peter Bramley established the advantage of using a single phytoene desaturase gene (bacterial CRTI), rather than introducing multiple plant desaturases (Romer et al., 2000). The combined activity of PSY and CRTI leads to the formation of lycopene, which is a red compound, its colour stemming from its undecaene chromophore, as is well established in tomato fruit. However, lycopene has never been observed in any rice transformant and different genetic backgrounds. Instead, α- and β-carotene are found together with variable amounts of oxygenated carotenoids (xanthophylls), such as lutein and zeaxanthin. The carotenoid pattern observed in the grain's endosperm revealed that the pathway proceeded beyond the end point expected from the enzymatic action of the two transgenes alone. A detailed analysis of the underlying mechanism has been published (Schaub et al., 2005). The findings are explained in some detail below (Figure 2).

Fig. 2. Filling a biosynthetic gap: Pathway elements in green are functional in wild-type rice grains. Thus the GGPP precursor molecule is being synthesized and lycopene can be cyclized. Elements in blue, including the blue box, are effectively absent. Introduction of the enzymes phytoene-synthase and the bacterial desaturase CRTI fills the biosynthetic gap created by the absence of the blue elements.
The explanation is that enzymes further down the pathway, such as lycopene cyclases (LCYs) and α- and β-carotene hydroxylases (HYDs), are still being produced in wild-type rice endosperm, while PSY and one or both of the plant carotene desaturases —phytoene desaturase (PDS) and ζ-carotene desaturase (ZDS)— as well as the cis-trans isomerases, namely ζ-carotene cis-trans Isomerase (Z-ISO; Chen et al., 2010) and carotene cis-trans isomerase (CRTISO; Isaacson et al., 2002; Park et al., 2002; Yu et al., 2011) are not. Synthesis of lycopene by PSY and CRTI in transgenic plants provides the substrate for these downstream enzymes and consequently enables the formation of the observed products.
The fact that a PSY transgene alone is sufficient for phytoene accumulation but does not lead to desaturated products (Burkhardt et al., 1997) is evidence for the absence of at least one active desaturase, namely PDS. Similarly, the expression of CRTI alone did not result in any coloured compounds in the rice endosperm, because of the lack of PSY activity. As stated above, the advantage of the CRTI desaturase lies in the fact that it can perform the entire reaction sequence from phytoene to lycopene on its own, while plants employ two desaturases and two cis-trans isomerases to achieve the same outcome. This reduces the number of transgenes required to only two.
The need for CRTI apparently conflicts with the presence of PDS and ZDS transcripts in wild-type endosperm, as shown by quantitative RT-PCR analyses. This could be due to low level presence of the enzyme rather than its mRNA. Due to the low-level expression, the complex reaction mechanisms of PDS and ZDS, and the unavailability of radioactive carotene substrates, the investigations were done using a transgenic approach rather than in vitro reactions. The tissue-specific expression of the PDS/ZDS system, instead of CRTI, in rice endosperm resulted in the formation of coloured carotenoids, showing that the rice endosperm provides the complex requirements for the activity of the plant desaturases.
The primary sequence of CRTI is unrelated to the plant-type desaturases. Its structure has been partially resolved and the reaction mechanism investigated (Schaub et al., 2012). Clearly, CRTI is simpler than plant-type desaturases. CRTI employs molecular oxygen directly as an electron acceptor, while the plant enzyme utilizes plastoquinone for this purpose, and is thus linked to and dependent on complex redox-chains (Beyer et al., 1989; Mayer et al., 1990; Nievelstein et al., 1995). This electron path is also indirectly linked to molecular oxygen as the terminal electron acceptor via an oxidase identified through the immutans mutation of Arabidopsis (for review, see Kuntz, 2004). This redox pathway is especially important in non-green carotenoid-bearing tissues, while the photosynthetic electron transport is thought to play an analogous role in chloroplasts. Moreover, CRTI does not form poly-cis-configured intermediates, as plant desaturases do (Bartley et al., 1999), and therefore, cis-trans isomerases (cf Fig. 2) are not required. CRTI is also capable of introducing all four double bonds in one step.
Clearly, lycopene cyclase activity relies on the expression of the respective rice genes in the endosperm, just as the occasional formation of xanthophylls, catalyzed by the divergent class of β-carotene hydroxylases (for a review, see Tian and DellaPenna, 2004). In all rice genetic backgrounds tested so far, complementation with these activities is not required to proceed down the pathway. Moreover, the activity of rice LCYs is obviously not rate limiting, since lycopene does not accumulate. Thus, Golden Rice is yellow because of the activity of intrinsic rice cyclases.
The beginnings of Golden Rice
Golden Rice: The First Generation
The first breakthrough in the development of Golden Rice was the result of a collaboration between Peter Beyer and Ingo Potrykus, and was obtained around Easter 1999 (Ye et al., 2000). This paper provided the proof that β-carotene could be produced in the rice grain. At the time it became evident already that only phytoene synthase and carotene desaturase (CRTI) were needed to get the pathway going, while lycopene cyclase was not required. These initial experiments were carried out with a Japonica (round grain) rice cultivar. Later, this was also achieved in Indica (long grain) cultivars (Hoa et al. 2003), a finding that has been confirmed many times over the years, by crossing the trait into a number of varieties by breeding.
With the proof of concept in hands, the scientists immediately proceeded to develop ways to improve the production and accumulation of carotenoids in the seed, as it was recognised that at the levels attainable at the time (1.6 μg/g) Golden Rice would not be able to cover the daily provitamin A requirements of the target population in the absence of a more varied diet. While some population strata in SE Asia do consume more varied diets, many of the poorest do not, and in fact, in some rural populations rice makes up more than 80% of their daily caloric intake.
These efforts led to the development of what we could call the first generation of Golden Rice (after the proof of concept), also known as GR1. This version only contained the phytoene synthase (Psy) gene from daffodil and the carotene desaturase (CrtI) gene from the bacterium Pantoea ananatis (previously known as Erwinia uredovora). Further, in this early version both transgenes were expressed only in the rice endosperm (by placing the genes under the control of the endosperm-specific gt1 promoter). The levels of carotenoids obtained in the field amounted to an average of 6 μg/g (about 4 times higher than the prototype), probably due the availability of large numbers of transformation events to select from and the use of the tissue-specific gt1 promoter to drive CRTI expression, while the constitutive 35S promoter had been used in the proof-of-concept prototype.
A new Golden Rice generation
The first generation of Golden Rice showed that it was possible to produce provitamin A in rice grains, but it was recognised that to combat vitamin A deficiency more higher β-carotene levels would be required. As only two biosynthetic transgenes are required in the process, the logical approach was to identify the bottleneck of the biosynthetic pathway and fine-tune the enzymatic activities of the two gene products involved, phytoene-synthase (PSY) and carotene-desaturase (CRTI).
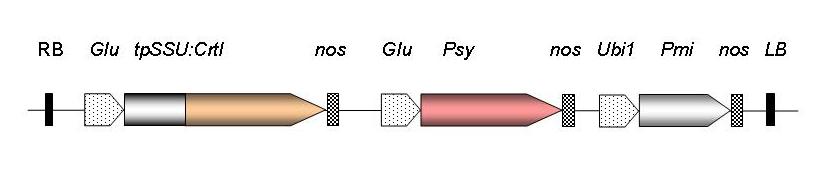
Fig 3: Gene construct used to generate Golden Rice. RB, T-DNA right border sequence; Glu, rice endosperm-specific glutelin promoter; CrtI, carotene desaturase from Pantoea ananas; tpSSU, pea ribulose bis-phosphate carboxylase small subunit transit peptide for chloroplast localisation; nos, nopaline synthase terminator; Psy, phytoene synthase gene from Narcissus pseudonarcissus (GR1) or Zea mays (GR2); Ubi1, maize polyubiquitin promoter; Pmi, phosphomannose isomerase gene from E. coli for positive selection (GR2); LB, T-DNA left border sequence.
In multi-step biosynthetic pathways there is generally a rate-limiting step that controls the flux through the whole pathway. This can be overcome by either increasing the amount of the rate-limiting enzyme or by using one that is more active. It was established that in this case was PSY and not CRTI (Al-Babili et al., 2006). Experimentation with PSY genes from different sources identified the maize and rice genes as the most efficient in rice grains (Paine et al., 2005), a result that has was confirmed later at the enzyme level (Welsch et al.,2010). This led to the second generation of Golden Rice lines, often referred to as GR2, capable of accumulating up to 37 μg/g carotenoids, of which 31 μg/g was β-carotene, as compared to the first generation, where only 1.6 βg/g were obtained. (see also Al-Babili and Beyer, 2005).
Given that bioconversion of β-carotene from Golden Rice is a very efficient process, as highlighted on the homepage of this website, a typical diet containing GR2 has a great potential to help alleviate vitamin A deficiency-induced diseases.

The image clearly shows the progress made since the proof-of-concept stage of Golden Rice. The new generation, also known as GR2 contains β-carotene levels that will provide adequate amounts of provitamin A in children's diets in SE Asia.
Publications
Literature cited
- Al-Babili S, Beyer P (2005) Golden Rice – five years on the road – five years to go? TRENDS in Plant Science 10:565-573.

- Al-Babili S, Hoa TTC, Schaub P (2006) Exploring the potential of the bacterial carotene desaturase CrtI to increase the β-carotene content in Golden Rice. J Exp Bot 57:1007-1014.
- Bartley G, Kumle A, Beyer P, Scolnik P (1993) Functional analysis and purification of enzymes for carotenoid biosynthesis expressed in photosynthetic bacteria. Methods Enzymol 214:374-385.
- Bartley GE, Scolnik PA, Beyer P (1999) Two Arabidopsis thaliana carotene desaturases, phytoene desaturase and zeta-carotene desaturase, expressed in Escherichia coli, catalyze a poly-cis pathway to yield pro-lycopene. Eur J Biochem 259:396-403.
- Beyer P, Al-Babili S, Ye X, Lucca P, Schaub P, Welsch R, Potrykus I (2002) Golden Rice: Introducing the beta-carotene biosynthesis pathway into rice endosperm by genetic engineering to defeat vitamin A deficiency. J Nutr 132:506S-510.
- Beyer P, Kroncke U, Nievelstein V (1991) On the mechanism of the lycopene isomerase/cyclase reaction in Narcissus pseudonarcissus L. chromoplasts. J Biol Chem 266:17072-17078.
- Beyer P, Mayer M, Kleinig H (1989) Molecular oxygen and the state of geometric isomerism of intermediates are essential in the carotene desaturation and cyclization reactions in daffodil chromoplasts. Eur J Biochem 184:141-150.
- Burkhardt P, Beyer P, Wunn J, Kloti A, Armstrong G, Schledz M, von Lintig J, Potrykus I (1997) Transgenic rice (Oryza sativa)endosperm expressing daffodil (Narcissus pseudonarcissus) phytoene synthase accumulates phytoene, a key intermediate of provitamin A biosynthesis. Plant J 11:1071-1078.
- Chen Y, Li FQ, Wurtzel ET(2010) Isolation and characterization of the Z-ISO gene encoding a missing component of carotenoid biosynthesis in plants. Plant Physiol 153:66-79.
- Hoa TTC, Al-Babili S, Schaub P, Potrykus I, Beyer P (2003) Golden indica and japonica rice lines amenable to deregulation. Plant Physiology 133:161-169.
- Isaacson T, Ronen G, Zamir D, Hirschber J (2002) Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of β-carotene and xanthophylls in plants. Plant Cell 14:333-342.
- Mayer M, Beyer P, Kleinig H (1990) Quinone compounds are able to replace molecular oxygen as terminal electron acceptor in phytoene desaturation in chromoplasts of Narcissus pseudonarcissus L. Eur J Biochem 191:359-363.
- Nievelstein V, Vandekerchove J, Tadros M, Lintig J, Nitschke W, Beyer P (1995) Carotene desaturation is linked to a respiratory redox pathway in Narcissus pseudonarcissus chromoplast membranes. Involvement of a 23-kDa oxygen-evolving-complex-like protein. Eur J Biochem 233:864-872.
- Paine JA, Shipton CA, Chaggar S, Howells RM, Kennedy MJ, Vernon G, Wright SY, Hinchliffe E, Adams JL, Silverstone AL, Drake R (2005) A new version of Golden Rice with increased provitamin A content. Nature Biotechnology 23:482-487.

- Park H, Kreunen SS, Cuttriss AJ, DellaPenna D, Pogson B (2002) Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation, and photomorphogenesis. Plant Cell 14:321-332.
- Rabbani S, Beyer P, Lintig Jv, Hugueney P, Kleinig H (1998) Induced beta-carotene synthesis driven by triacylglycerol deposition in the unicellular alga Dunaliella bardawil. Plant Physiology 116:1239-1248.
- Romer S, Fraser PD, Kiano JW, Shipton CA, Misawa N, Schuch W, Bramley, PM (2000) Elevation of the provitamin A content of transgenic tomato plants. Nature Biotechnology 18:666-669.
- Schaub P, Al-Babili S, Drake R, Beyer P (2005) Why is Golden Rice golden (yellow) instead of red? Plant Physiology 138:441-450.

- Schaub P, Yu Q, Gemmecker S, Poussin-Courmontagne P, Mailliot J, McEwen AG, Ghisla S, Al-Babili S, Cavarelli J, Beyer, P (2012) On the structure and function of the phytoene desaturase CRTI from Pantoea ananatis, a membrane-peripheral and FAD-dependent oxidase/isomerase. PLoS ONE 7:e39550.
- Schreier PH, Seftor EA, Schell J, Bohnert HJ (1985) The use of nuclear-encoded sequences to direct the light-regulated synthesis and transport of a foreign protein into plant chloroplasts EMBO J 4:25-32.
- Tian L, DellaPenna D(2004) Progress in understanding the origin and functions of carotenoid hydroxylases in plants. Arch Biochem Biophys 430:22-29
- Welsch R, Arango J, Bär C, Salazar B, Al-Babili S, Beltrán J, Chavarriaga P, Ceballos H, Tohme J, Beyer P (2010) Provitamin A accumulation in cassava (Manihot esculenta) roots driven by a single nucleotide polymorphism in a phytoene synthase gene. Plant Cell 22:3348-3356.
- Ye X, Al-Babili S, Klöti A, Zhang J, Lucca P, Beyer P, Potrykus I (2000) Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 287:303-305.

- Yu Q, Ghisla S, Hirschberg J, Mann V, Beyer P (2011) Plant carotene cis-trans isomerase CRTISO: A new member of the FADred-dependent flavoproteins catalyzing non-redox reactions. J Biol Chem 286:8666-8676.

